
Mass numbers of typical isotopes of Francium are 223. Electron configuration of Francium is 7s1.
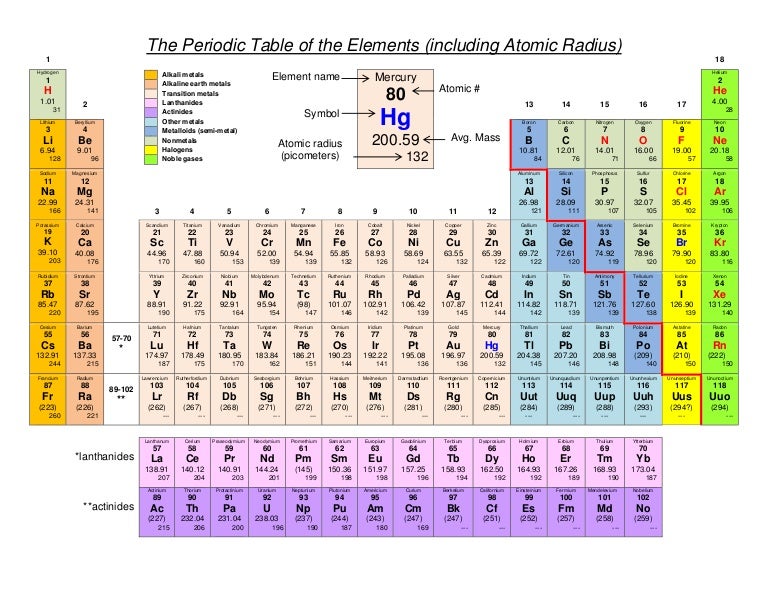
In the periodic table, the elements are listed in order of increasing atomic number Z. How is francium listed on the periodic table? The atomic radius of a chemical element is a measure of the distance out to which the electron cloud extends from the nucleus. It must be noted, atoms lack a well-defined outer boundary. The atomic radius of Francium atom is 260pm (covalent radius). How big is the atomic radius of francium? The explosion would be so dangerous and would be fatal. If Francium were to touch water, it would cause a huge explosion. What would happen if you touched francium?įrancium is a radioactive metal, also known as an alkali metal because it has one valence electron. 2Fr +2 H2O = H2 + 2FrOH.Ĭesium (Cs), tucked in the lower left hand corner of the table, has the largest known atoms. Hence, Caesium is known as the biggest atom.Ī piece of francium is blown apart when treated with water giving off hydrogen and forming francium hydroxide with generation of a lot of heat. By this logic you can get confused by Francium being the biggest atom but Francium is radioactive and is highly unstable. This tends to increase or decrease the atomic size of the elements. The atomic radius gets smaller because the electron orbit fills up to form an octet and for each of the additional electron, a proton is added to the nucleus, pulling all the electron shells closer around the nucleus. Helium is the atom having the smallest size. Thus, helium is the smallest element, and francium is the largest.
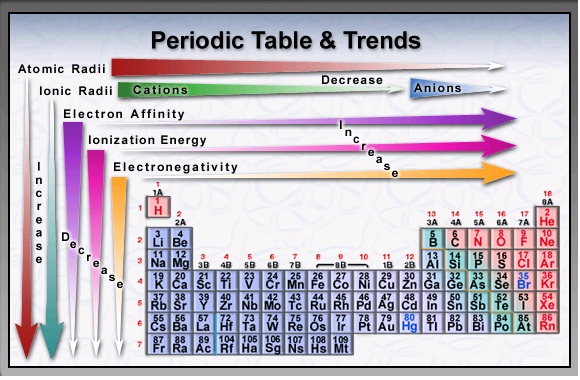
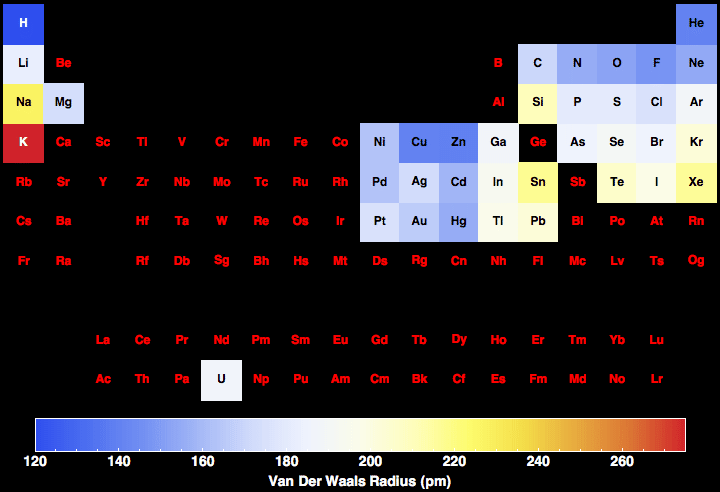
As can be seen in the figures below, the atomic radius increases from top to bottom in a group, and decreases from left to right across a period. 6: Francium has one of the largest, heaviest atoms of all elements.Ītomic radii vary in a predictable way across the periodic table. Pure hydrogen is a colorless, odorless, tasteless gas that is nontoxic but highly flammable.


 0 kommentar(er)
0 kommentar(er)
